A lot of the initial fuel cell development work was conducted in the 1970s at NASA. Fuel cells were used in the Apollo mission as on board power supply devices, which operated on hydrogen as the fuel. The exhaust water generated as a by-product from these systems was used by the astronauts for drinking. The conceptual development and lab-scale research on fuel cells is at least a hundred years older. Another name for Hydrogen Fuel Cells is PEMFC, which stands for Proton exchange membrane fuel cells.
Design of a FC system
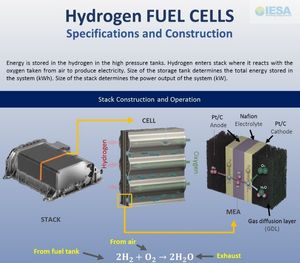
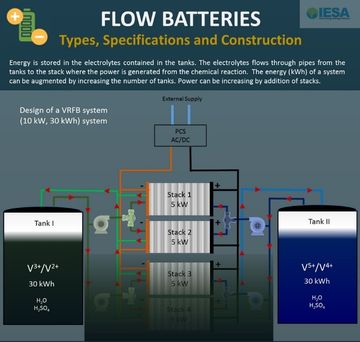
The design of a FC (fuel cell) system is very similar to a flow battery, which was described in the previous issue of ETN. There is a fuel tank, which contains the required fuel such as Hydrogen (for PEMFC) or methane, natural gas or methanol. The fuel flows to the stack which is the heart of the system and where the generation of electricity takes place. The crucial difference from flow batteries or any other secondary battery is that fuel cells are unidirectional systems. They are designed to consume fuel and produce electricity but not vice versa. Hence, a FC is not an energy storage technology, but rather an energy conversion device. In a PEMFC, the hydrogen from the tank reacts with the oxygen absorbed from air in the stack to generate electricity. The only by-product of this reaction is water as shown in the chemical equation.
The variations
As in case of Li-ion batteries or flow batteries, there are many types of fuel cells. The prominent ones other than PEMFC are SOFC (solid oxide fuel cells), PAFC (phosphoric acid fuel cells), AFC (alkaline fuel cells), MCFC (molten carbonate fuel cells) and DMFC (direct methanol fuel cells). Depending on the type of FC, the standard operating temperature can vary between 100°C to 1000°C. Other than that, the compactness (kW/kg) of the stack can also vary greatly. As a result, certain type of FCs are more adapted to certain applications from a practicality point of view. For example, the stack of a PEMFC is very compact, its operating temperature is conveniently low (< 120°C), and the stacks are highly scalable between 200 W to 150 kW. This makes it the FC of choice for transportation applications, home backup as also for portable electronics. However, for very large stationary systems (10s of MW), generally SOFCs, PAFCs or MCFCs are more practical.
Hydrogen (H2) as a fuel
One of the key advantages of using hydrogen as a fuel is the fact that it produces only water upon combustion. It does not produce any CO2. The other advantage is that it is extremely lightweight. One kg of H2 stores 39 kWh of energy. With the electrical conversion efficiency of a PEMFC being 55%, we can generate 21 kWh of electrical energy from one kg of H2. By comparison, one kg of petrol has 13 kWh of energy but the efficiency of an ICE engine is only 20%. So we can generate only 2.6 kWh of energy from 1 kg of petrol, which is 7X lower than H2. One the other hand, one of the major challenges for H2 is that it takes up a lot of volume. It is not a major impediment, but there is definitely lots of room for improvement. The storage pressure that hydrogen tanks are able to hold has increased from 150 bars (in 2005) to 700 bars (in 2018). This means 5 times more H2 stored in the same volume than previously. Inspite of these improvements, in current state of the art prototypes, a 140 L tank still stores only 5 kg of H2. On the aspect of compact storage, other approaches under development are metal hydride storage, chemical storage and cryogenic storage tanks.
Design of the PEMFC Stack
A stack consists of a few hundred cells stacked together in series. In each cell, the hydrogen enters from one side and air from the other side. The two gases are not allowed to mix and are kept separated by a proton conducting membrane. The most popular choice for this membrane material is Nafion, which is the same material used in Vanadium Flow Batteries (VRBs). Each such cell produces a voltage of 0.7 V, which is obviously not enough for any application. However, by putting hundreds of cells in series in a stack, a practically useful voltage is generated. In case of PEMFCs, which operate at a relatively low temperature a special catalyst is needed at the electrodes. The best catalyst material is Platinum (Pt) which is coated on carbon (C) particles to form the electrodes. This abbreviated as Pt/C. The use of platinum is the main reason for the high cost of the PEMFC stack. In the pie chart, this shows up as the high fractional cost (76%) of the MEA (membrane electrode assembly). In the last 10-15 years, a major focus of industrial and academic R&D has been to minimize the quantity of Pt required (or Pt loading in mg/cm2) without compromising the performance. The other major focus has been on improving the compactness of the stack. Between 2008 and 2019, the power density of the PEMFC stack has been doubled from 0.83 kW/kg to 2.1 kW/kg. The volume reduction is also two times with the power density increasing from 1.4 kW/L to 3.1 kW/L. In the transportation sector, these efforts have been led by Toyota and Honda for cars, Plug Power for material handling equipment (MHE) and by Ballard and Hydrogenics for FC buses.
Applications of PEMFCs
One of the challenges of using PEMFCs for transportation applications is its response time for varying power requirement. During driving, the power requirement (kW) is constantly changing depending on whether the vehicle is accelerating or cruising. Additionally, during deceleration, the energy generated through regenerative braking needs to be stored somewhere. Being a uni-directional device, a PEMFC is incapable of storing the energy. These issues are solved by including a small battery pack (1-2 kWh) along with the FC system. For a mid-sized car, 1 kg of H2 provides approximately 100 km of driving range. Current PEMFC cars have a capacity of holding 5-6 litres of H2, which gives a driving range of 500+ km. Beyond cars and buses, in recent times the applicability of PEMFC to trains and aeroplanes is being tested. The first train running on fuel cells developed by Alstom is already operational in Germany. Note specifically, the missing overhead electrical lines. With the first use of PEMFC being for space applications, it would make sense for them to find new uses in non-land based applications. In this direction, attempts at powering small aircraft using PEMFCs are ongoing at Boeing, the projects notably being in early development stage. Smaller PEMFCs are also available as home backup systems (1-5 kW) as well as portable power units ( Electrolyzers: The missing half )
Unlike petrol or diesel, the fuel required for PEMFCs is not readily obtainable by mining. It has to be produced, distributed and made available when any user is in need of it. This requirement is fulfilled by electrolyzers. These industrial scale machines produce H2 and O2 via water electrolysis using electric power as input. Some companies manufacturing such systems are Hydrogenics, ITM Power, Frames and Proton-on-site. Electolyzers are available in a different sizes ranging from 10 kW – 10 MW that corresponds to a hydrogen generation capacity of 4.5 kg – 4500 kg per day. The idea of having distributed generation via electrolyzers at hydrogen fuelling stations is very attractive, as it could potentially save on fuel transportation costs. Some stations of this type are already operational in California, Japan and some Scandinavian countries. PEMFCs have attained a high level of technological maturity and their longevity and reliability has been demonstrated in several applications. Increased scale of manufacturing has the potential to bring down the cost manifold, inspite of the challenges posed by the usage of an expensive Platinum catalyst in the electrodes. Nevertheless, as the hydrogen fuel production and distribution network continues to grow in many regions of the world supported by various government initiatives, the time for PEM fuel cells will arrive sooner than later.